Ethanol in breath analysis
Discover here a practical use case for our NDIR Ethanol gas sensor.
Ethanol is the main component of alcohol in alcoholic beverages, and its presence in exhaled air is directly related to the concentration of alcohol in the blood.
Ethanol in exhaled air theory
The measurement of ethanol (ethyl alcohol) in exhaled air is relevant in many fields, including road safety, blood alcohol control, industrial safety and medical research. Ethanol is the main component of alcohol in alcoholic beverages, and its presence in exhaled air is directly related to the concentration of alcohol in the blood.
The concentration of ethanol in exhaled breath is closely correlated with the concentration of alcohol in blood, allowing law enforcement, health care professionals and safety officers to quickly and accurately determine whether a person has exceeded the legal blood alcohol limit.
SCIENCE
How to measure ethanol in breath with infrared spectroscopy?
Infrared spectroscopy detection relies on an in-depth electronic analysis of an infrared radiation beam passing through a breath sample to determine the concentration of ethanol by identifying molecules based on their light absorption properties. Using this technique, spectrometers are capable of measuring the amount of ethanol present in the breath and displaying the results.
A light source emits infrared radiation, which is directed into a measuring chamber and subsequently measured by a detector. As the breath sample is analyzed in the chamber, certain molecules, including ethanol, absorb a portion of the radiation, leading to a reduction in the intensity of the optical signal. This enables the deduction of the ethanol concentration.
However, more than a single compound may absorb radiation at one or more similar wavelengths to ethyl alcohol, but the IR spectroscopy technology combined to algorithms is able to identify these particular compounds thanks to their signature wavelengths, and not take them into account.

IR Spectroscopy has the benefit to differentiate between alcohol level in lungs and in upper tracks, including residual alcohol in the breath. When you drink a glass of alcohol, molecules are present in your mouth, trachea and esophagus. When you take a test in a alcohol breathalyzer based in semiconductor or fuel cell technology, it could appear as a positive result not representative of your real alcohol level concentration in your blood. By measuring the concentration over the time at a high frequency, IR spectroscopy helps to detect false positive results.
On the graph on the left, we can observe how IR spectroscopy effectively distinguishes between alcohol levels in the lungs and those in the upper respiratory tract. IR spectroscopy continuously analyzes the optical signal and can accurately detect the presence of alcohol. The specific shape of a “normal” breath is represented as a “tray,” so any other shape is not indicative of a correct measurement, including alcohol present in the mouth.
SCIENCE
Infrared technology in breath testing technology
Alcohol regulation varies across European countries, making it essential to create a portable technology accessible to both consumers and professionals. This technology is designed to accurately determine the precise amount of alcohol present in a given breath sample.
Currently, most breath alcohol test devices available for European consumers rely on either fuel cells or semiconductor sensors. However, these options have inherent drawbacks in terms of precision, limited lifespan, and the need for frequent calibration and replacement.
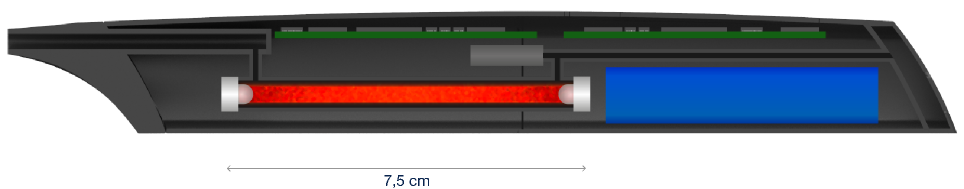
To overcome these limitations, Olythe has introduced a groundbreaking solution: the first miniature and compact infrared breathalyzer specifically designed for consumer use. This innovative breathalyzer integrates the same technology employed by law enforcement to validate alcohol concentration levels.
Breath alcohol test devices for consumers in the European market are subject to regulation under the EN16280 standard. To meet this standard, breathalyzers must achieve a defined precision level of below 20 parts per million (ppm) and be capable of performing 75 continuous tests. This poses a significant challenge for compact devices like OCIGO, as they need to excel in both measurement precision and energy management.
The accuracy of the NDIR (non-dispersive infrared) system used in these devices depends mainly on the path length, as explained by Beer-Lambert’s law. Reducing the overall size from 200 mm (typical for probationary breath analyzers) to less than 50 mm was a real challenge.
Additionally, miniaturizing the NDIR system presents several other challenges for the measuring cell, including the need for low power and high-performance infrared components, precise and stable thermoregulation, and robust signal processing.
In the past, IR Spectroscopy technology and its precision and reliability were mainly accessible to those in Law Enforcement. However, Olythe, after dedicating six years to research and development, successfully created OCIGO, an electronic breathalyzer using infrared spectroscopy technology.
Olythe managed to miniaturize this complex technology to fit into your pocket while offering the same level of precision as IR Spectroscopy, but at a more affordable price comparable to fuel-cell technology.
